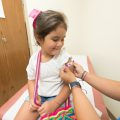
Adar Poonawalla, CEO of the Serum Institute of India, applauded the government’s choice to enable all adults to receive Coronavirus booster doses at privatized vaccination facilities beginning Sunday. According to the vaccine businessman, people who wanted to travel were figuring it impossible to do so without a third shot because numerous nations have set limitations on those who have not taken a booster shot.
Price of the Booster shot
The third jab will not be free for most adults, unlike the booster doses provided for healthcare workers, frontline employees, and those over 60. Mr Poonawala told NDTV that Covishield will cost Rs 600 plus taxes (the same as before) and Covovax will cost Rs 900 plus taxes once licensed as a booster.
Last month, India’s pharma authority gave Serum Institute’s COVID-19 vaccination Covovax restricted emergency usage authorization for the 12 to 17-year-old age group, subject to specific circumstances. Covovax was developed through a technology transfer from Novavax and was conferred categorical marketing authorization by the European Medicines Agency in December 2017. The World Health Organization also allotted it emergency consumption listing in December 2020.
Statement of the Government
“Currently underway free vaccination program via government immunization facilities for first and second doses, as well as Safety Dose to Healthcare Workers, Frontline Workers, and the 60+ population will proceed and be accelerated,” a government notification said this morning when declaring the decision.
According to the report, about 96 percent of the country’s citizens aged 15 and over have received at least one dose of COVID-19 vaccination, while about 83 percent have obtained both doses. On March 16, India began immunizing youngsters between 12 to 14 years. The government has yet to decide whether or not to vaccinate children under the age of 12 years, and the health ministry has repeatedly said that further immunization needs and population inclusion for vaccination are continually being assessed.